Latest advances in technology has decreased the entry price of nanoscopy. Read this article to find out more.
Written by our Biophotonics Team Leader Peter Collins.
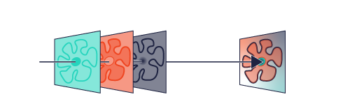
At Laser 2000 (UK) we have been busy playing around with our new low cost motorised inverted epi-fluorescence microscope platform (MVR) by adding our SPINDLE module, which allows users to select engineered point spread functions by adding phase masks, such as a double helix or tetrapod, into the imaging arm, for applications such as STORM, PALM etc. Further details in the article below.
We are looking for partners to help test and provide feedback, so please contact me if you are in the UK/Ireland and are interested in either upgrading an existing microscope frame or using our modular microscope.
1. Background
Due to the diffraction of light, traditional light microscopy is unable to resolve structural details of less than ~200nm laterally and ~500nm axially. After decades of gradual improvements, various ‘super-resolution’ light microscopy (SRLM or nanoscopy) techniques have emerged, featuring a 2 to 10 times improvement in resolution. These techniques are typically available to groups that can construct advanced microscopes from components or shared facilities that purchase complete systems from the ‘big four’ or some specialist suppliers. Due to the expense of this equipment, Biologist will need to book time in order to use them. The solutions we offer are over three times lower cost than comparable systems, allowing access to equipment for individuals or facilities that want to free up resource.
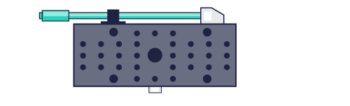
SPINDLE – 3D Localisation and particle tracking
The SPINDLE easily attaches to any microscopes camera port, with interchangeable phase masks and filter holders you can switch from widefield to single molecule localisation microscopy.

With a library of engineered Phase Masks you can control depth and precision, by engineering the Point Spread Function (PSF) of the microscope. The accompanying software 3DTRAX is an easy-to-use Fiji/ImageJ plugin that simplifies 3D localisation analysis by automating 3D calibration, drift correction, super-resolution image reconstruction and particle tracking
“We expect that the DH-PSF optics will become a regular attachment on advanced microscopes, either for super-resolution 3D imaging of structure, or for 3D super-resolution tracking of individually labeled bio-molecules in cells or other environments.”
Professor W.E Moerner | Nobel Laureate |Stanford University
As can be seen below, by inserting a phase mask into the pupil plane of the microscope, we can modify the PSF from the standard Airy Disk, to that of a Double Helix PSF.
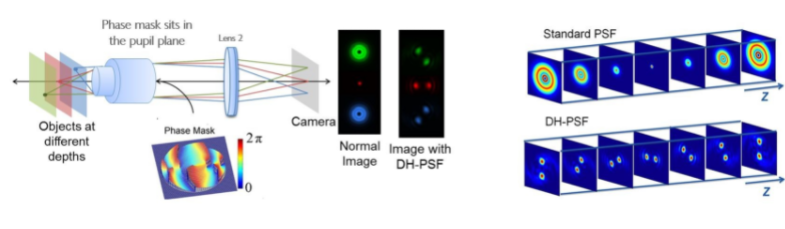
Using the Double Helix phase mask, we can determine the lateral location of the emitter based on the centre point of the two lobes, and the axial position of the emitter based on the angle the two lobes make. This allows for extended depth of over five times.
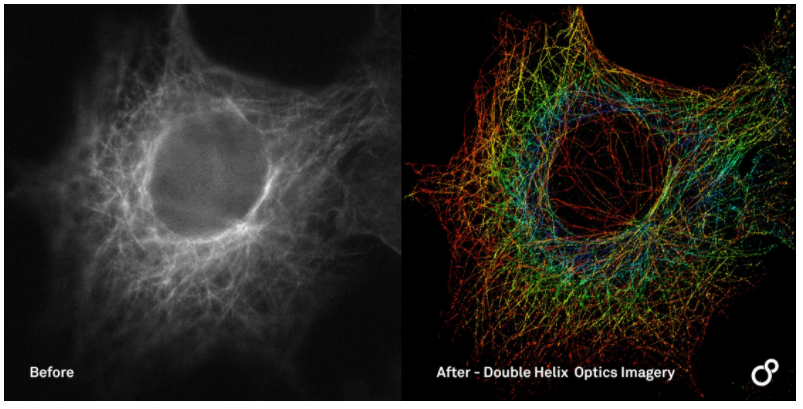
The above greyscale image is a standard widefield image where the depth of the field is limited by the airy disk. By applying the DH-PSF & localisation techniques, we can see that the depth of field improves AND we are able to visualise the result with a depth-encoded colour map.
For this image we have a 65um field of view, showing 2.3um of depth. The average lateral precision is less than 20nm and the average axial precision is less than 25nm.
2. Application example – 3D Light sheet imaging with engineered PSF
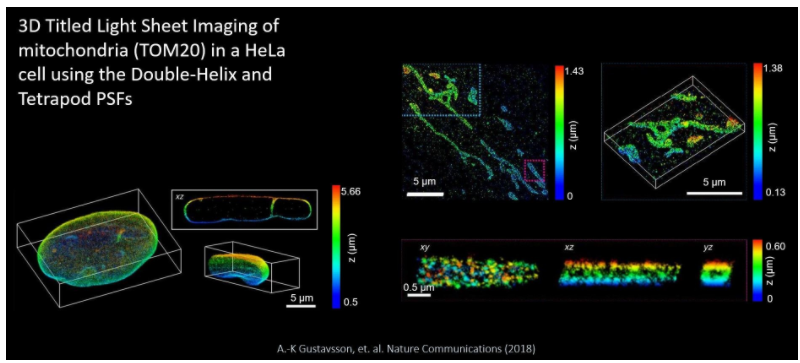
Representative image volumes are presented here, where the TILT3D system was used to image mitochondria in a HeLa Cell. At the top right we have a 3D super-resolution reconstructions of mitochondria (TOM20) in a HeLa cell. the DH-PSD was used for imaging of both single molecules and fiducial beads.
On the bottom right are xy, xz and yz views of the mitochondrion shown in the magenta rectangle above, revealing the hollow cylinder structure of the mitochondrial outer membrane. finally, the bottom left is a 3D super-resolution reconstruction of the entire nuclear lamina (lamin B1) in a HeLa cell. Imaging of single molecules and fiducial beads was performed with the DH-PSF and a 6um tetrapod PSF, respectively. The xz view sows a 1.3um thick y-slice through the cell, where lamin meshwork enveloping an intranuclear channel is visible.
If you would like to test and book a demo of any of the products mentioned in this article please feel free to contact me directly on [email protected] or send me a direct message.