Compact FLIM and FCS Upgrade Kit for Laser Scanning Microscopes
- FLIM, FRET, and FCS in one turn-key system
- Compact, easy-to-use and maintenance-free kit, customized to all major LSMs in various configurations for unlimited flexibility
- Highest sensitivity with up to 4 detection channels
- Fluorescence lifetimes from < 100 ps up to µs
- Advanced and user-friendly data analysis software with multiple analysis tools
- Options for anisotropy measurements and deep-tissue FLIM imaging
- NEW: rapidFLIMHiRes – visualise dynamic processes with ultra fast FLIM imaging and outstanding 5 ps time resolution
Applications
The compact FLIM and FCS upgrade kit enables multiple time-resolved applications with Laser Scanning Microscopes (LSMs), such as:
- Time-Resolved Fluorescence
- rapidFLIMHiRes – Redefining standards for dynamic FLIM imaging
- Fluorescence Lifetime Imaging (FLIM)
- Phosphorescence Lifetime Imaging (PLIM)
- Fluorescence Correlation Spectroscopy (FCS)
- Fluorescence Lifetime Correlation Spectroscopy (FLCS)
- Fluorescence Cross-Correlation Spectroscopy (FCCS)
- Foerster Resonance Energy Transfer (FRET)
- Pulsed Interleaved Excitation (PIE)
- Laser Cutting/Ablation
- Pattern Matching Analysis
- Time-Resolved Photoluminescence (TRPL)
- TRPL Imaging
- Antibunching
- Anisotropy
Confocal Laser Scanning Microscopes (LSMs) are widely used tools in biochemistry, cell biology, and other related life sciences. The capabilities of these microscopes can be further enhanced by using time-resolved techniques, granting following advantages:
- Lifetime FRET for quantitative measurements of FRET efficiency
- Time-resolved imaging reveals environmental parameters such as ion concentrations or pH
- Lifetime measurements are independent from fluorophore concentration
- Separation of molecules with spectrally overlapping emission by lifetime fingerprinting
- Reduced number of needed detectors – one detector is sufficient for simultaneous detection of different fluorophores based on their specific lifetimes by pattern matching
- Discrimination of fluorescence light against elastic and Raman scattering and other background contributions by temporal resolution
- Decay time as a further parameter enhances the accuracy of analytical measurements
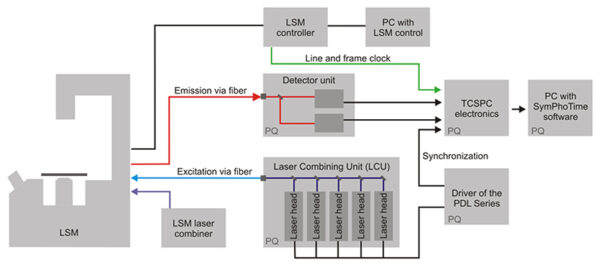
The Upgrade Kit is an external addition to the LSM and grants easy-to-use, versatile, and comfortable performance without time-consuming adjustments. The turn-key setup consists of three major parts: picosecond pulsed excitation, single molecule sensitive detection, and Time-Correlated Single Photon Counting (TCSPC) data acquisition.
Flexible excitation system
All samples containing commonly used fluorophores can be examined with the LSM Upgrade Kit. For this purpose, the excitation subsystem consists of a pulsed diode laser driver of the PDL Series and different laser heads with pulses in the picosecond time regime (additional CW mode is available as an option).

The available wavelengths range from 375 to 900 nm. The laser heads are integrated along with multiple optical components in one Laser Combining Unit (LCU) for comfortable handling and coupling into an optical fiber enabling single or multicolour excitation. The sample background is significantly reduced due Directly coupled laser diode head from the LDH Seriesto the narrow excitation spectra of the lasers.

The repetition rate and laser power can be adapted via the laser driver to the fluorescence lifetime which minimises bleaching of the sample. As an alternative to pulsed diode lasers, especially for multi-photon excitation schemes, short pulse laser systems such as Titanium:Sapphire lasers can be integrated as well.

Excellent timing with picosecond resolution
Fluorescence lifetimes down to a few picoseconds or even up to milliseconds for phosphorescence and luminescence studies are easily resolvable with the LSM Upgrade Kit. This broad measurement range covers almost all samples that are analysed in life as well as material sciences. Time-resolved microscopy requires the registration of not only the photons themselves, but also their position in time and, for imaging, in space. The ideal technique for that purpose is the Time-Tagged Time-Resolved (TTTR) data acquisition developed by PicoQuant, which is a variation of the classical method of Time-Correlated Single Photon Counting (TCSPC). Using TTTR data acquisition vastly different measurement procedures, like FLIM, lifetime based FRET, FCS, or even coincidence correlation (“antibunching”) can be performed, based on just one fundamental data format. The TTTR format is supported by all available TCSPC electronics from PicoQuant. Different modules with up to eight independent TCSPC channels for fast and parallel detection are available which provide highest data throughput, multi-stop possibility, and low dead time to realise short acquisition times.

Single photon sensitive detection for highest sensitivity
To ensure optimal experimental conditions, four different detector types are available for the LSM upgrade kit:
- Hybrid PMT, the best allrounder for FLIM, FCS, and NDD deep tissue imaging
- PDM SPAD, the fastest detector for FLIM and FCS
- SPCM-AQRH SPAD, the highest sensitivity detector best for FCS
- Photomultiplier tubes (PMTs) of the PMA series, the economic variant for FLIM
Hybrid PMTs and SPADs offer highest sensitivity as needed for single molecule experiments and are suited for, e.g., cellular FLIM and FCS studies at low fluorescence intensities. The detectors differ in their efficiency, temporal resolution, spectral range, or active area. Thus, the choice of the detector depends on several factors such as the targeted application or interface to the microscope for confocal or NDD (non-descanned) detection.
Confocal and NDD detection
The detectors can be attached in confocal mode using a pinhole and fitting to all detector types, as well as in non-descanned detection (NDD) for multi photon excitation.
In the standard confocal mode, the detectors are connected via an optical fiber to the fiber exit port of the microscope. In this confocal configuration set-ups with up to 4 detectors are available. Integrated filter holders allow quick change of emission filters in order to adapt to different experimental conditions and fluorophores.
The non-descanned configuration (NDD) is used in systems with a multi-photon laser and enables deep-tissue FLIM. In this case one or two detectors (PMTs or Hybrid PMTs) are connected to the LSM via a large core liquid light guide. The light guide is either mounted to a suited NDD port of the microscope or, alternatively, a specially developed dove-tail adapter collects the fluorescence directly above the objective. The latter approach features a very high collection efficiency due the light collection very near to objective and the high fluorescence transmission through the liquid light guide. In case of the Olympus FluoView FV1000/1200 MPE system it is also possible to use up to four internal NDD standard PMT detectors of the LSM for FLIM measurements.
Multichannel detection unit
To offer greatest flexibility and multiple applications in one system, the multichannel detection unit allows for parallel confocal, polarization, and NDD measurements with up to four detection channels for multi color FLIM, FRET, deep tissue FLIM imaging, auto- and crosscorrelation (FCS, FLCS, FCCS, FLCCS) as well as anisotropy studies. Several detection configurations are available:
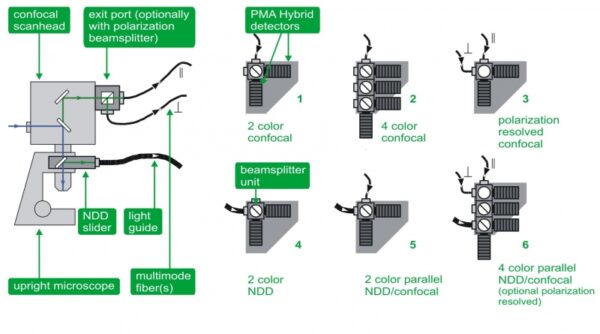
- Dual-channel confocal detection for FLIM, FRET, auto- and crosscorrelation (FCS, FLCS, FCCS, FLCCS)
- Four-channel standard confocal detection for FLIM, FRET, auto- and crosscorrelation (FCS, FLCS, FCCS, FLCCS)
- Dual channel polarization resolved confocal detection for anisotropy imaging
- Dual channel NDD set-up for deep tissue FLIM and FRET
- Parallel dual channel set-up enabling confocal and non-descanned detection for dual color FLIM, FRET, deep tissue FLIM imaging, auto- and crosscorrelation (FCS, FLCS, FCCS, FLCCS)
- Parallel four channel set-up enabling confocal as well as non-descanned detection and optionally polarization measurements for multi color FLIM, FRET, deep tissue FLIM imaging, auto- and crosscorrelation (FCS, FLCS, FCCS, FLCCS), and anisotropy.
- All options are also available for inverse microscope setups.
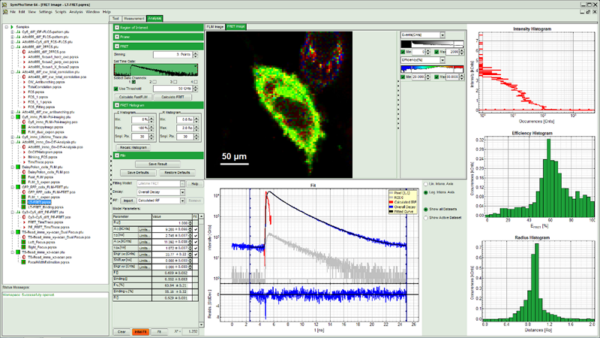
Intuitive software
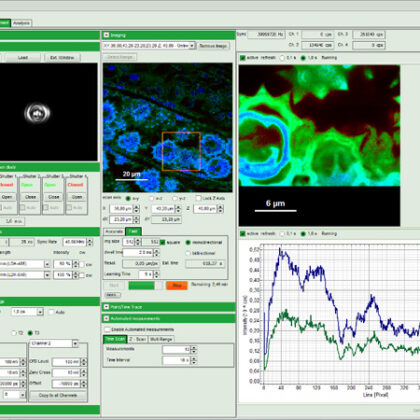
The system software SymPhoTime 64 provides multiple, easy-to-use acquisition and analysis tools for all kind of samples. Based on the sophisticated data collection and handling, the system software SymPhoTime 64 supports a multitude of methods, such as Fluorescence Lifetime Imaging (FLIM), Förster Resonance Energy Transfer (FRET), Fluorescence Correlation Spectroscopy (FCS), Fluorescence Lifetime Correlation Spectroscopy (FLCS), intensity time trace, burst analysis, lifetime histogramming, and anisotropy, to name only a few.
SymPhoTime 64 data handling maintains a transparent data structure where all derived data is stored in one workspace, including a log file to keep track of all measurement and analysis steps. Image data can be processed further or exported to standard formats. A large number of algorithms for those methods are already integrated in the software, providing a analysis platform for ready-to-publish data. At the same time, SymPhoTime 64 offers enhanced flexibility for the integration of novel, cutting edge algorithms by the user. A dedicated scripting language interface allows to modify and expand the analysis routines according to the experimental needs.
A specially developed interface between the SymPhoTime 64 and the LSM software (Nikon and Leica) strongly facilitates data acquisition and enables straightforward recording of, e.g., FLIM images up to 4096 × 4096 pixels, time series FLIM, z-stacks as well as single and multipoint measurements for FCS. Online previews of the main parameters (Fast FLIM, FCS, time-traces, or TCSPC histograms) allow to quickly optimise data acquisition.
| Supported LSMs | |
|---|---|
| Nikon |
|
| Olympus |
|
| Scientifica |
|
| Zeiss |
|