Time-resolved Confocal Fluorescence Microscope with Super-resolution Capability
- Complete confocal STED system based on inverted microscope body
- Optical resolution below 50 nm
- Excitation at 640 nm and optionally with additional lasers at 595 nm and 660 nm
- Up to 6 truly parallel detection channels using SPADs or Hybrid-PMTs
- Supports gated STED (gSTED) and gSTED-FCS
- Piezo scanning for 2D- and 3D-(lifetime) imaging and accurate point positioning
- Advanced easy-to-use data acquisition and analysis software SymPhoTime 64
- Upgrade options for simultaneous AFM/FLIM/STED measurements
- Supports all other methods available for the MicroTime 200, i.e., FLIM, FCS, FCCS, FLCS, FRET, etc.
- FLIMbee galvo scanner add-on with outstanding flexibility in scanning speed and excellent spatial precision
- Scanning FCS available via line scan in x using FLIMbee galvo scanner
The MicroTime 200 STED is a time-resolved confocal microscope with single molecule sensitivity and super-resolution capabilities. Hence, numrous applications are possble with this instrument, including:
- Stimulated Emission Depletion Microscopy (STED) / gated STED
- Single Molecule Spectroscopy / Detection
- Time-Resolved Fluorescence
- Fluorescence Lifetime Imaging (FLIM)
- Phosphorescence Lifetime Imaging (PLIM)
- Fluorescence Correlation Spectroscopy (FCS)
- Fluorescence Lifetime Correlation Spectroscopy (FLCS)
- Foerster Resonance Energy Transfer (FRET)
- Dual-focus Fluorescence Correlation Spectroscopy (2fFCS)
- Scanning FCS (sFCS)
- Pulsed Interleaved Excitation (PIE)
- Fluorescence Anisotropy (Polarization)
- Pattern Matching Analysis
- Time-Resolved Photoluminescence (TRPL)
- TRPL Imaging
- Antibunching
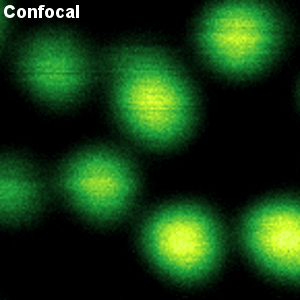
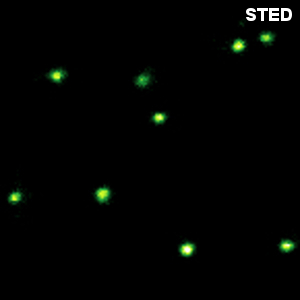
In recent years, super-resolution microscopy has gained more and more attention. It has now evolved beyond the stage of development and permits to investigate biological systems that were formerly obscured by the diffraction limit of light. One of the most popular techniques for super-resolution imaging is Stimulated Emission Depletion (STED) microscopy. STED is usually performed with confocal microscopes and is therefore ideally suited to be added to the MicroTime 200. The integration of STED into the system has been driven towards highest robustness and ease-of-use. The system permits to perform STED microscopy without lengthy alignment preparations while still having the choice to modify the system and use the full capability of the open microscopy platform MicroTime 200.

Less than 50 nm optical resolution
STED microscopy uses the principle of stimulated emission depletion. After exciting fluorophores in the laser focus, a second, donut-shaped focus of a laser with longer wavelength is used to actively de-excite the molecules in the periphery via stimulated emission. In the MicroTime 200 STED, the donut is created using a so-called EASYDOnut phase plate. It is inserted into the beam path and changes the STED laser focus to a donut-shape, while leaving the excitation laser unaffected. This simple implementation makes spatial alignment of the two laser beams, which emerge from the same optical fiber, unnecessary and yields a spatial resolution of less than 50 nm. Due to the time-resolved data acquisition principle of the MicroTime 200 STED, it is additionally possible to apply time-gates to all measured data. This gated STED (or gSTED) approach leads to an enhanced resolution in images and a reduced observation volume for FCS.

Choice of excitation wavelengths
The excitation system of the MicroTime 200 STED is build around a dedicated laser combining unit that integrates the excitation lasers for STED, and the STED laser itself. The standard excitation wavelength is 640 nm, which can optionally be combined with additional lasers at 595 nm and 660 nm for dual species STED imaging. Due to the high power of the STED laser at 765 nm, special care was taken to ensure the user’s safety when operating the instrument. A second laser combining unit with additional pulsed diode lasers can also be attached to the system for non-STED applications such as FLIM, FRET or FCS.
The laser power and repetition rate can be flexibly adjusted by the multichannel laser driver PDL 828 “Sepia II”, which even allows to address several lasers in parallel enabling advanced techniques like Pulsed Interleaved Excitation (PIE). The time delay between the excitation pulse and the STED pulse is adjustable for highest flexibility and best STED resolution.

Scanning Technologies
The great versatility of the MicroTime 200 platform is complemented by the FLIMbee galvo scanner which can provide scanning speeds ranging from very slow to fast while maintaining high precision. This high degree of flexibility in speed allows for applications ranging from Phosphorescence Lifetime Imaging (PLIM) to fast fluorescence lifetime measurements using rapidFLIM. Furthermore, with its high precision and sensitivity, the FLIMbee scanner is optimally suited for super-resolution microscopy via STED, enabling imaging down to the single molecule level.
A MicroTime 200 equipped with a FLIMbee scanner is a good choice for Single Molecule Detection (SMD) methods such as spFRET, PIE-FRET, (STED-)FCS, FLCS, FLCCS, dual-focus FCS (2fFCS), and even anisotropy measurements. Additionally, Two-Photon Excitation (TPE) with descanned and non-descanned detection is possible.
The core of the FLIMbee galvo scanner consists of three high precision oscillating mirrors with excellent linearity, repeatability and low drift. The two y-axis galvo mirrors ensure that the laser beam is stationary at the entrance of the objective. This mirror configuration minimises vignetting of the image field and ensures a constant focal volume over a wide scan range. The FLIMbee scanner provides a minimal pixel size of 10 nm when using a 100x objective.
Use of the standard piezo scanner is recommended for applications requiring light from the UV (255 to 400 nm) and NIR (1100 to 1400 nm) spectral regions or when pixel sizes smaller than 10 nm are desired.

Detection subsystem with single photon sensitivity
In the MicroTime 200 STED, scanning is facilitated through a piezo table optionally combined with a high precision PiFoc element for 3D imaging. The choice of piezo scanning ensures a high repositioning accuracy and stability, which is essential for high quality STED images. The system can be configured for up to six individual detection channels. For the deep-red emission of the STED dyes depleted at 765 nm, SPADs are typically used, since they feature a high detection efficiency of up to 70% in this spectral range.

Gated STED with picosecond resolution
Data acquisition is performed using time-correlated single photon counting (TCSPC) using PicoQuant’s unique time-tagged time-resolved (TTTR) data acquisition mode with picosecond resolution. The acquired TTTR data can not only be used for regular STED imaging, but also to perform gated STED (gSTED) measurements. The advantage of TTTR data acquisition mode is that it allows to perform vastly different measurement procedures besides STED, such as FLIM, FCS, or even coincidence correlation (“antibunching”), based on just one fundamental data format. The TTTR format is supported by all available TCSPC electronics from PicoQuant.
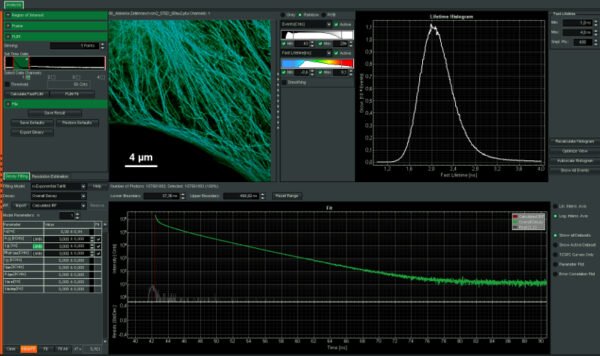
Intuitive data acquisition and analysis
SymPhoTime 64, the MicroTime 200 STED system software, features dedicated STED data acquisition and analysis protocols. For example, preset time-gating is integrated in the measurement preview, and flexible time gates for higher STED resolution can be set during data analysis. The unique fluorescence pattern matching approach can be used to separate multiple species from data recorded with the same STED laser wavelength.
FLIM, FRET, FCS, and more
The MicroTime 200 STED can not only be used to perform STED and STED-FCS, but also supports all other measurement and analysis procedures available for the MicroTime 200. This includes FLIM, FCS, FCCS, FLCS, FRET, PIE-FRET, or intensity time-traces, to name just a few.